The compound HB can be represented as 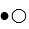 where
where 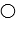 represent B- and
represent B- and  represents H+. Given this, ___ would represent the conjugate acid of HB and ____ would represent the conjugate base.
represents H+. Given this, ___ would represent the conjugate acid of HB and ____ would represent the conjugate base.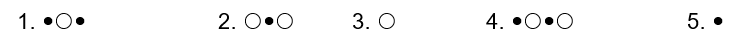
A) 1,2
B) 1,5
C) 3,4
D) 1,3
E) None of the above.
Correct Answer:
Verified
Q8: What is the conjugate base of thiocyanic
Q9: The dihydrogen phosphate ion, H2PO4 - ,
Q10: Ethanol (CH3CH2OH), like water, can behave both
Q11: What is the conjugate base of HSO4-?
A)
Q12: Methanol is described by the following skeleton
Q14: Which of the following is the conjugate
Q15: Which of the following represents a conjugate
Q16: A solution is known to have a
Q17: What is the [H3O+] concentration of a
Q18: A solution of weak base is added
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents