What can be correctly concluded from the fact that the following acid-base reaction proceeds to the right, as written?
NH2-(aq) + HSO4-(aq) 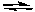 NH3(aq) + SO42-(aq)
NH3(aq) + SO42-(aq)
A) NH3 is a stronger base than NH2-.
B) NH3 is a stronger acid than HSO4-.
C) NH3 is a weaker acid than NH2-.
D) NH3 is a weaker base than HSO4-.
E) NH3 is a weaker acid than HSO4-.
Correct Answer:
Verified
Q29: If the strength of the following acids
Q30: Explain the following observation: Water becomes acidic
Q31: Which of the following is the weakest
Q32: Arrange the following bases in order of
Q33: Which of the following statements is true?
A)
Q35: Which of the following statements is true?
A)
Q36: The hydride ion, (H-), is a stronger
Q37: Which of the following structural factors is
Q38: Which of the following is the best
Q39: Of the following, which is the strongest
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents