Ammonia and the ammonium ion form a conjugate acid-base pair.
NH3(aq) + H2O(l) 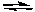 NH4+(aq) + OH-(aq)
NH4+(aq) + OH-(aq)
Calculate the pH of 0.10 M NH3 if Ka for the NH4+ ion is 5.6 x 10-10.
A) 4.7
B) 5.1
C) 5.7
D) 8.9
E) 11.1
Correct Answer:
Verified
Q66: The salt NaF is dissolved in water.
Q67: When NH4Cl is dissolved in water the
Q68: What is the value of Kb for
Q69: The base-ionization constants for three bases, A(OH),
Q70: There are many ways of "fluoridating" water.
Q72: Calculate the pH of a solution prepared
Q73: What is the OH- ion concentration in
Q74: What is the H3O+ ion concentration in
Q75: HA is a weak monoprotic acid with
Q76: Which of the following solutions would have
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents