Which of the following initial concentrations of reactants and products will cause the reaction to proceed to the right in order to establish equilibrium?
2 NO2(g) 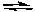 2 NO(g) + O2(g) Kc=3.4*10 -F 7 (at 200
2 NO(g) + O2(g) Kc=3.4*10 -F 7 (at 200  C)
C) 
A) 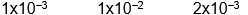
B) 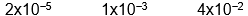
C) 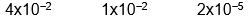
D) 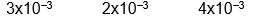
E) none of these
Correct Answer:
Verified
Q16: Which is the correct equilibrium constant expression
Q17: Assume that the equilibrium constant for the
Q18: For the reaction shown below, at equilibrium
I2(g)
Q19: Based on the information given in the
Q20: Under which set of conditions must the
Q22: If at any moment in time, we
Q23: Assume that the reaction quotient, Qc, for
Q24: If the equilibrium constant for the following
Q25: For the following reaction:
2 NOCl(g) 
Q26: Suppose that the reaction quotient, Qc,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents