Which of the following changes will shift the following equilibrium to the right?
AgCl(s) 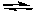 Ag+(aq) + Cl-(aq) H = 65.4 kJ/molrxn
Ag+(aq) + Cl-(aq) H = 65.4 kJ/molrxn
A) adding more AgCl(s)
B) adding NaCl
C) adding AgNO3
D) increasing the temperature
E) None of the above will shift the equilibrium to the right.
Correct Answer:
Verified
Q84: A solution is prepared in which the
Q85: If a solution has a Mg2+ ion
Q86: A solution is prepared in which the
Q87: A 50.0 mL solution of 0.015 M
Q88: In which of the following will the
Q89: Which of the following statements is correct?
A)
Q90: Ag2SO4(s) is in equilibrium with silver and
Q91: What is the concentration of Ag+ (mol/L)
Q92: Approximately how many grams of Ag2CO3 will
Q94: A solution is 0.10 M in Ca(NO3)2.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents