refer to the phase diagram shown below.
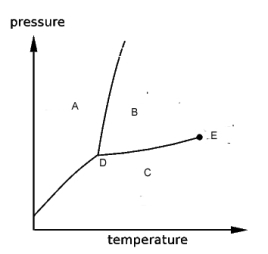
-If the temperature and pressure are changed from the values at point B to the values at point C, the substance will change its phase from
A) liquid to gas.
B) liquid to solid
C) gas to solid.
D) solid to liquid.
E) gas to liquid.
Correct Answer:
Verified
Q16: A liquid is at its equilibrium vapor
Q17: The boiling point of HF is lower
Q18: In most cases, reading from left to
Q19: Which statement isn't consistent with the information
Q20: refer to the phase diagram shown
Q22: refer to the phase diagram shown
Q23: The best explanation for the fact that
Q24: Which of the following will be most
Q25: Which alcohol should be most soluble in
Q26: Carboxylic acids with the general formula CH3(CH2)nCO2H
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents