Calculate Hrxn° for the following reaction from the data given below. 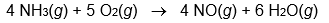
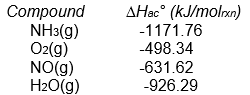
A) -105.5 kJ/molrxn
B) -905.5 kJ/molrxn
C) -1274.2 kJ/molrxn
D) -1996.2 kJ/molrxn
E) none of the above
Correct Answer:
Verified
Q22: The bond strength between C and a
Q23: When carbon is burned in air
Q24: Which of the following is an
Q25: Determine the change in enthalpy for the
Q26: Use enthalpies of atom combination to
Q28: Calculate
Q29: Both ethanol (CH3CH2OH) and methanol (CH3OH) have
Q30: The following reaction occurs when sucrose
Q31: What is the sign of the
Q32: What is the absolute value of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents