The bond-type triangle can be used for
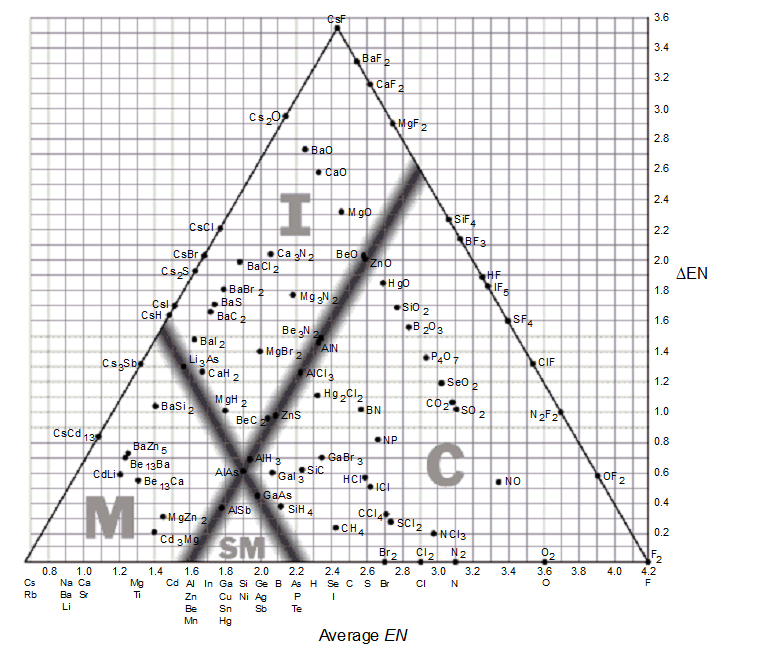
-Based on the bond-type triangle, which of these covalent compounds has the greatest ionic character?
A) NCl3
B) SCl2
C) ICl
D) HCl
E) SiC
Correct Answer:
Verified
Q35: Predict the product of the reaction between
Q36: An element X forms a compound with
Q37: In Chapter 4 we were able to
Q38: If the difference in electronegativity between two
Q39: Compare and contrast (what is the same
Q41: The bond-type triangle can be used for
Q42: The bond-type triangle can be used for
Q43: The bond-type triangle can be used for
Q44: The bond-type triangle can be used for
Q45: The bond-type triangle can be used for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents