Assume that the XF3 molecule has the following Lewis structure. 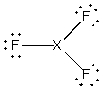
The molecule could be:
A) boron trifluoride, i.e., X = B
B) nitrogen trifluoride, i.e., X = N
C) chlorine trifluoride, i.e., X = Cl
D) phosphorus trifluoride, i.e., X = P
E) more than one of the above
Correct Answer:
Verified
Q10: Which of the following elements would form
Q11: Nitrous oxide (N2O) is an anesthetic commonly
Q12: Which element would form XF5 for which
Q13: If X is a 3rd row element
Q14: If the XF6- ion has the Lewis
Q16: Which of the following elements would form
Q17: If there are two pairs of nonbonding
Q18: What is element X in the following
Q19: Which of the following species contains only
Q20: Which of the following would be the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents