The "zwitterion" form of the amino acid glycine is:
H3N+CH2CO2-. The properties of the various amino acids depend heavily on the presence (or absence) of charge on the side chain. Consider the amino acid arginine, for example, which has the following Lewis structure:
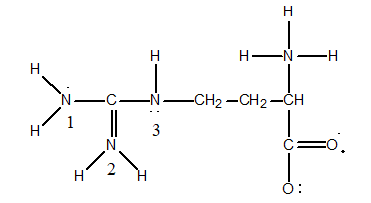 Determine the Formal Charge on the nitrogens labeled 1, 2 and 3.
Determine the Formal Charge on the nitrogens labeled 1, 2 and 3.
A) N(1) = 0, N(2) = +1, and N(3) = 0
B) N(1) = 0, N(2) = -1, and N(3) = 0
C) N(1) = +1, N(2) = 0, and N(3) = +1
D) N(1) = -1, N(2) = 0, and N(3) = -1
E) N(1) = +1, N(2) = 0, and N(3) = -1
Correct Answer:
Verified
Q42: Which of the following elements has the
Q43: Which of the following lists the elements
Q44: Which of the following is the best
Q45: In which of the following compounds is
Q46: Use formal charge to decide which of
Q48: Our response to the anthrax scare was
Q49: Which element will combine with sulfur to
Q50: In which compound are the valence electrons
Q51: What is the distribution of electrons around
Q52: What is the distribution of the oxygen
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents