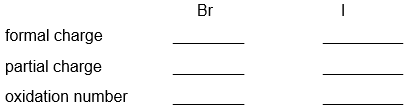
Partial charge, formal charge and oxidation number are all used to describe the arrangement of electrons or charge associated with atoms within a molecule. Determine the formal charge, partial charge and oxidation number on both atoms in IBr. Give a one sentence description for each of the three calculations describing what the calculation tells you. Which of these three calculations most accurately describes the distribution of charge between I and Br?
Correct Answer:
Verified
Form...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q84: Which of the following molecules is (are)
Q85: Which of the following factors influence the
Q86: CO2 is a non-polar molecule but SO2
Q87: Bond dissociation energies are defined as the
Q88: The best way for the body to
Q90: The partial charge on F in the
Q91: If there is a single pair of
Q92: What would be the distribution of electrons
Q93: Which element would form a neutral XF3
Q94: The compound XCl3 has a trigonal pyramidal
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents