Use the following molecular orbital diagram to predict which of the following has the largest bond order: 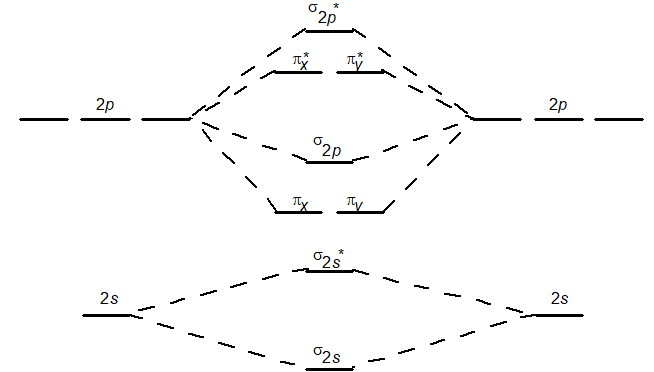
A) NO+
B) NO
C) NO-
D) NO2-
E) all of the above have the same bond order
Correct Answer:
Verified
Q90: The partial charge on F in the
Q91: If there is a single pair of
Q92: What would be the distribution of electrons
Q93: Which element would form a neutral XF3
Q94: The compound XCl3 has a trigonal pyramidal
Q96: Use the same molecular orbital diagram to
Q97: Which type of hybrid orbitals are used
Q98: In which of the following compounds is
Q99: What is the hybridization of the central
Q100: What is the hybridization of the sulfur
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents