Which of the following selection rules for quantum numbers is incorrectly stated?
A) n is any integer greater than or equal to zero.
B) l is any integer between zero and n - 1.
C) ml is any integer between -/ and +/.
D) ms is either + 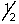 or -
or - 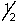 .
.
E) All of the above selection rules are correctly stated.
Correct Answer:
Verified
Q49: Which element is represented by the following
Q50: Which of the following atoms would have
Q51: Which is a legitimate set of n,
Q52: Which set of n, l, ml and
Q53: Which of the following sets of n,
Q55: Which of the following quantum numbers is
Q56: Which set of quantum numbers can be
Q57: How many electrons can be placed in
Q58: Which of the following atomic orbitals doesn't
Q59: What is the orbital designation for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents