The amount of solute in each of the beakers shown below is represented by the number of black dots. The volume is shown on the side. In which beaker does the solute have the highest concentration?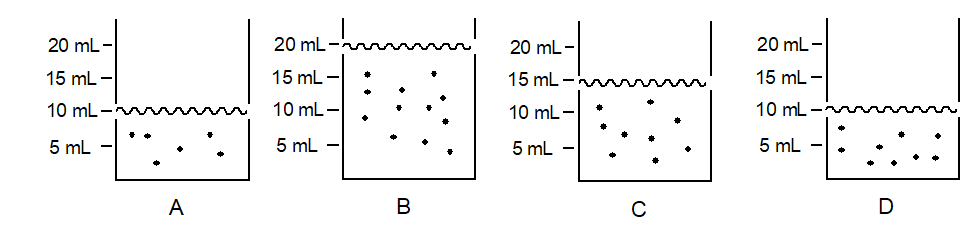
A) Beaker A
B) Beaker B
C) Beaker C
D) Beaker D
E) The solute concentration is the same in each beaker.
Correct Answer:
Verified
Q99: A sample of sucrose (C12H22O11) that weighs
Q100: The following liquids don't mix with water.
Q101: If a beaker that contains 250 mL
Q102: A student was given three pieces
Q103: Retailers purchase gasoline by weight so they
Q105: What is the molarity of 0.0250 liters
Q106: What weight of HNO3 is present in
Q107: How many moles of Na2SO4 are in
Q108: How many grams of NaNO3 are required
Q109: Calculate the molarity of a solution prepared
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents