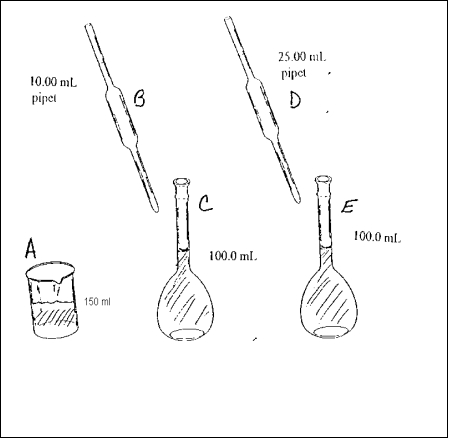
 The following four are based on the diagram above which describes the following lab procedure. Ten mL of an initial solution is pipetted into a volumetric flask. The 10.00 mL of initial solution is diluted to 100.0 mL with solvent to form the stock solution. Twenty five mL of the stock solution is then pipetted into another 100.0 mL volumetric flask and diluted to the 100.0 mL mark to form the final solution. The glassware in the diagram has been labeled with letters.
The following four are based on the diagram above which describes the following lab procedure. Ten mL of an initial solution is pipetted into a volumetric flask. The 10.00 mL of initial solution is diluted to 100.0 mL with solvent to form the stock solution. Twenty five mL of the stock solution is then pipetted into another 100.0 mL volumetric flask and diluted to the 100.0 mL mark to form the final solution. The glassware in the diagram has been labeled with letters.
-Which of the following statements correctly describes the relationship between moles of solute in the labeled containers?
A) A = C
B) A = B
C) D > C
D) D > E
E) C > E
Correct Answer:
Verified
Q112: Calculate the concentration of a solution prepared
Q113: What is the molarity of concentrated phosphoric
Q114: How many mL of 15 M NH3

