Carbon dioxide (CO2) is readily soluble in water, according to the equation CO2 + H2O H2CO3. Carbonic acid (H2CO3) is a weak acid. If CO2 is bubbled into a beaker containing pure, freshly-distilled water, which of the following graphs correctly describes the results?
A) 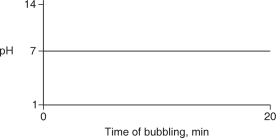
B) 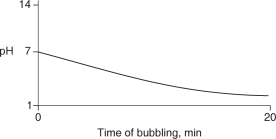
C) 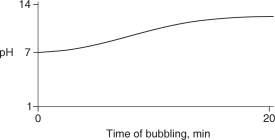
D) 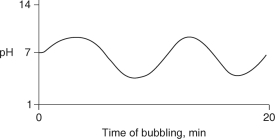
E) 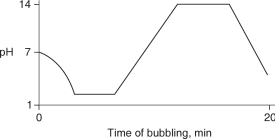
Correct Answer:
Verified
Q3: Which one of the atoms shown would
Q4: Which one of the atoms shown would
Q5: Which of the following pairs of atoms
Q6: Which of the following pairs of atoms
Q7: A given solution contains 0.0001(10-4) moles of
Q9: Carbon dioxide (CO2) is readily soluble
Q10: Which of the pairs of molecular structures
Q11: Which of the pairs of molecular structures
Q12: Identify the asymmetric carbon in this molecule:
Q13: For the following questions, match the labeled
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents