The radioactive decay of 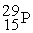 to
to 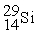 is an example of ________.
is an example of ________.
A) positron emission or electron capture
B) gamma emission or beta emission
C) alpha emission
D) beta emission
Correct Answer:
Verified
Q28: Which of the following statements about synthetically
Q29: What is the material, X, in
Q30: Bombardment of cadmium-113 with a(n) _ produces
Q31: The bombardment of 23Na with
Q32: A positron is produced in a nucleus
Q34: Phosphorus-30 undergoes a radioactive decay process
Q35: Before positron emission the n/p ratio of
Q36: In a stable heavy nucleus the neutron
Q37: Radionuclides which have too low of a
Q38: Calculate the neutron-to-proton ratio after beta decay
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents