The form of the expression for the equilibrium constant, Keq, for the reaction below is:
4 NH3 (g) + 5 O2 (g) 4 NO (g) + 6 H2O (g)
A) 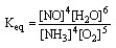
B) 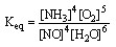
C) 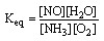
D) 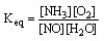
Correct Answer:
Verified
Q27: Given the following decomposition reaction: PCl5
Q28: For a reaction which has an equilibrium
Q29: All of the following factors may shift
Q30: What effect does a catalyst have on
Q31: If at equilibrium, reactant concentrations are slightly
Q33: The form of the expression for
Q34: At a given temperature, K =
Q35: Sulfur combines with hydrogen to form
Q36: Hydrogen gas reacts with iron(III) oxide
Q37: CO2 and H2 are allowed to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents