Multiple Choice
The ideal gas equation cannot be written as ________.
A) PV = nRT
B) P = 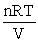
C) R = 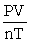
D) R = 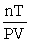
Correct Answer:
Verified
Related Questions
Q40: A 15.0 L cylinder was filled with
Q41: Calculate the density of butane (C4H10) in
Q42: Calculate the density of ammonia (NH3) gas
Q43: The density of a gaseous chlorofluorocarbon (CFC)
Q44: 6.80 L of CH4 gas at 0.980
Q46: The value of the ideal gas constant,
Q47: The temperature, in °C, occupied by 0.643
Q48: At what temperature in °C does 0.444
Q49: Calculate the volume of 12.0 g of
Q50: Which of the following is a correct
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents