If liquid ammonia and water were mixed, between which two atoms in these molecules would a hydrogen bond form?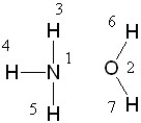
A) between 1 and 3
B) between 2 and 6
C) between 3 and 2, 4 and 2, 5 and 2, 1 and 6, and 1 and 7
D) Hydrogen bond formation is not possible between these two molecules.
Correct Answer:
Verified
Q46: Which of the following substances would be
Q47: Which of the following would have the
Q48: The anomalously high boiling point of water
Q49: In a liquid sample of NCl3, what
Q50: If liquid methyl amine and hydrofluoric acid
Q52: Which of the following molecules are capable
Q53: Which of the following types of crystalline
Q54: The crystal lattice sites in a solid
Q55: In which of the following types of
Q56: Which one of the following is classified
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents