Use a dashed line to illustrate hydrogen bonding between a methanol molecule and a hydrogen fluoride molecule.
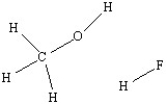
Correct Answer:
Verified
Q55: In which of the following types of
Q56: Which one of the following is classified
Q57: What would be the temperature change, in
Q58: A chocolate, caramel pecan piece of candy
Q59: The specific heat of lead is 0.13
Q60: The ClF molecule is polar. Use +
Q62: Predict the predominate intermolecular force (London force,
Q63: Predict the predominate intermolecular force (London force,
Q64: Predict the predominate intermolecular force (London force,
Q65: Predict the predominate intermolecular force (London force,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents