Which of the following is the correct "set-up" for the problem "How many grams of H2O will be produced from 3.2 moles of O2 and an excess of H2S?" according to the reaction:
2H2S + 3 O2 2H2O + 2SO2
A) 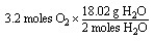
B) 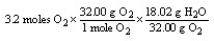
C) 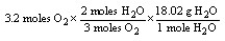
D) 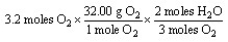
Correct Answer:
Verified
Q17: Given: 2 N2 + 5 O2
Q18: How many moles of Al are
Q19: Given the following equation, 2 N2
Q20: How many moles of CO2 will
Q21: Given: 2 Na + O2
Q23: The "set-up" below for the problem
Q24: In the following reaction, how many
Q25: For the reaction: 2 P +
Q26: In the reaction: 4HPO3 + 12C
Q27: How many moles of H2O will
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents