The "set-up" for the problem "How many oxygen atoms are there in 4.0 moles of N2O4?" below is correct, except for the use of letters rather than numbers in the conversion factors. What are the correct numerical values of A and D, respectively?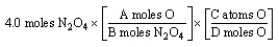
A) 4 and 4
B) 1 and 4
C) 4 and 1
D) 4 and 6.02 1023
Correct Answer:
Verified
Q43: How many moles of nitrogen, N2, are
Q44: How many grams of carbon are present
Q45: Which of the following is the correct
Q46: The "set-up" for the problem "What is
Q47: Which of the following is the correct
Q49: Which of the following is the correct
Q50: The number of moles of O atoms
Q51: How many atoms of H are present
Q52: How many atoms are present in 0.65
Q53: Calculate the number of potassium ions present
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents