The atomic orbital depicted below would be found in a ________ subshell.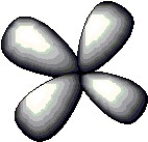
A) 3p
B) 2s
C) 5d
D) 4f
Correct Answer:
Verified
Q19: The shape of an orbital is most
Q20: Which statement about orbitals is incorrect?
A) Different
Q21: What is the maximum number of electrons
Q22: What is the maximum number of orbitals
Q23: The atomic orbital depicted is a(n) _
Q25: Which of the following is a representation
Q26: Which of the following subshell notations for
Q27: In an atom with many electrons, which
Q28: After the 5s subshell of an atom
Q29: Indicate the missing words in the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents