What is the atomic number of an isobar of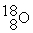 In which there is an equal number of all three types of subatomic particles present?
In which there is an equal number of all three types of subatomic particles present?
A) 8
B) 9
C) 10
D) 11
Correct Answer:
Verified
Q30: Choose the correct chemical symbol for an
Q31: Isotopes of a given element _.
A) have
Q32: The nucleus of atom A contains 20
Q33: What is the mass number of a
Q34: Iron is element number 26. This means
Q36: Which pair of neutrons and protons correspond
Q37: Which of the following pairs of atoms
Q38: Which of the following represents a pair
Q39: Which of the following properties for the
Q40: Chlorine, which exists in nature in two
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents