The hypothetical element supposium exists in four isotopic forms. The relative masses and percentage abundances for these four isotopes are, respectively,
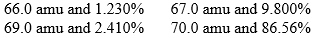
Calculate the atomic mass of supposium.
Correct Answer:
Verified
Q76: For each description on the left select
Q77: For each description on the left select
Q78: For each description on the left select
Q79: For each description on the left select
Q80: Fill in the chart below for each
Q82: Naturally occurring iron contains 5.82% of 54Fe,
Q83: The five naturally abundant isotopes of elemental
Q84: On a new atomic mass scale suppose
Q85: The hypothetical element athenium (Ah) occurs in
Q86: Determine the relative mass for the hypothetical
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents