What is the slow, rate-determining step, in the acid-catalyzed dehydration of 2-methyl-2-propanol?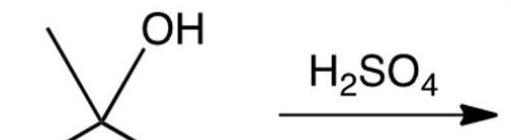
A) Protonation of the alcohol to form an oxonium ion.
B) Loss of water from the oxonium ion to form a carbocation.
C) Loss of a -hydrogen from the carbocation to form an alkene.
D) The simultaneous loss of a -hydrogen and water from the oxonium ion.
Correct Answer:
Verified
Q1: Carbon-carbon double bonds do not freely
Q2: What is the IUPAC name of the
Q3: Which of the following alkenes exhibit
Q4: Which of the following
Q5: Identify the major organic product expected from
Q7: Which of the following carbocations is(are) likely
Q8: Predict the major product of the following
Q9: In the dehydrohalogenation of 2-bromobutane, which conformation
Q10: Which of the following cannot undergo an
Q11: Which of the following would have the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents