Which one of the following monomers undergoes cationic polymerization most readily?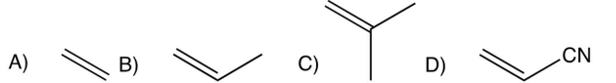
A)
B)
C)
D)
Correct Answer:
Verified
Q1: Which of the following is the monomer
Q3: Which one of the following monomers
Q4: The monomer used to make superglue is
Q5: Which one of the following is the
Q6: Identify the repeating unit in the polymer
Q7: The acid-catalyzed dimerization of isobutylene gives
Q8: The repeating unit of poly (methyl
Q9: Which one of the following monomers is
Q10: Which one of the following initiators
Q11: Which one of the following initiators
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents