The reaction of propene (C3CHCH2) with hydrochloric acid.
HCl + C3CHCH2
CH3CHClCH3
A proposed mechanism for this chemical reaction is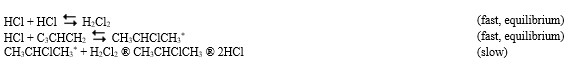
-Refer to Exhibit 18-3. If the first step were the slow step, what would be the predicted order of the reaction with respect to propene?
A) 0
B) 1
C) 2
D) Experimental data required to answer this question
E) none of the above
Correct Answer:
Verified
Q2: The initial rate data given below
Q3: The initial rate data given below
Q4: The initial rate data given below for
Q5: The initial rate data given below for
Q6: The initial rate data given below for
Q7: The initial rate data given below for
Q8: The reaction of propene (C3CHCH2) with hydrochloric
Q9: The reaction of propene (C3CHCH2) with hydrochloric
Q11: The reaction of propene (C3CHCH2) with hydrochloric
Q12: The following question(s) pertain to The
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents