Allotropic forms of an element that do not represent an element in its standard state will have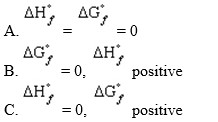
D) S°=0
E) None of the above
Correct Answer:
Verified
Q1: Calculate DS°rxn for the following
NH3(g) +
Q2: Calculate DS°rxn for the following
3NO2(g) +
Q3: pertain to the reaction CCl4(l)
Q5: A solid of mass m initially at
Q6: The constant that connects the entropy of
Q7: For a reversible isothermal process involving ideal
Q8: Copper has a specific heat capacity of
Q9: A 125.0 g piece of iron is
Q10: The decomposition of NO and NO2
Q11: Sublimation is the process of changing phases
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents