The wavefunction for the 2s orbital in hydrogen is given by 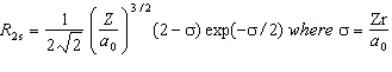 At what radius is there a node?
At what radius is there a node?
A) r = 0
B) r = 2/a0
C) r = a0
D) r = 2a0
E) there are no radial nodes in this function
Correct Answer:
Verified
Q1: A MO orbital for a heteronuclear diatomic
Q2: The molecule HeH+ has an antibonding MO
Q3: Oxygen is paramagnetic and has a bond
Q4: Which of the following combinations of atomic
Q5: Given that Zeff(2s) in Li is 1.26,
Q6: Rank from smallest to largest in terms
Q7: What do you predict of the electron
Q9: Of the following angular components for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents