Sodium perbromate can be synthesized via the following reaction that gives off Xenon gas.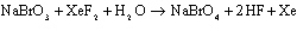
A reaction is started with 200.0g of NaBrO3, 250.0g of XeF2 and 100.0g of water. Assuming that the reaction goes to completion, and that all of the formed xenon gas is allowed to escape into the atmosphere, what will be the minimum mass of products and reactants remaining in the reaction vessel?
A) 173.4 g
B) 193.9 g
C) 376.0 g
D) 356.1 g
E) None of the above
Correct Answer:
Verified
Q5: Calcium carbide, CaC2, reacts with water
Q6: Ammonium perchlorate, NH4ClO4, is used with
Q7: Aluminum sulfide reacts with water to
Q8: Iron oxide can be reduced to
Q9: Acetonitrile, CH3CN, can be synthesized from
Q10: Which has a larger molar volume (the
Q11: Copper oxide ore, CuO, can be
Q12: A vanadium oxide contains 56.02% vanadium by
Q13: Caffeine has the molecular formula, C8H10N4O2. If
Q14: Triphenylene is an organic compound containing only
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents