Suppose the average thermal speed of molecules in a gas at room temperature is . If we "double" the gas's temperature (to ) , what is the average molecular speed now? Select the closest response.
A) About the same
B) 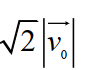
C) 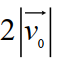
D) 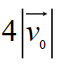
E) 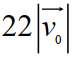
Correct Answer:
Verified
Q161: A solid ball and a hollow
Q162: Various objects are released from rest at
Q163: Various objects are released from rest at
Q164: An isolated star collapses so that its
Q165: A railroad car collides with a similar
Q167: If you rub your hands together, they
Q168: Is the specified change in the following
Q169: Is the specified change in the following
Q170: Is the specified change in the
Q171: Is the specified change in the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents