Suppose the interaction between two atoms has the effective potential energy curve shown below:
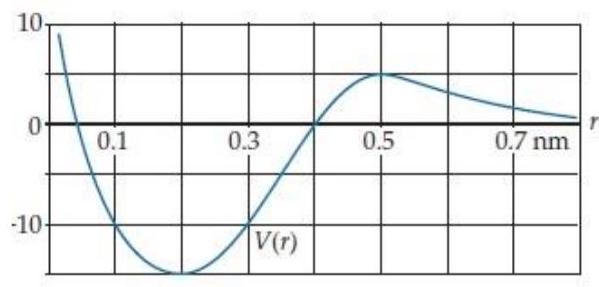
I have marked the vertical scale on this graph using an arbitrary energy unit we'll call bondits.
-Suppose instead we know that at the atoms are at rest at a separation of . What minimum energy is required to break the bond in this case?
A) Only a smidgen (less than a bondit)
B) 5 bondits
C) 10 bondits
D) 15 bondits
E) 20 bondits
F) The bond is already broken (or we can't form a bond) .
Correct Answer:
Verified
Q177: You and a classmate are discussing
Q178: Substance
Q179: Substance
Q180: Substance
Q181: Suppose the interaction between two atoms
Q183: Suppose the interaction between two atoms has
Q184: Suppose we put a
Q185: The thermal energy of a block
Q186: An egg will cook no more rapidly
Q187: A 100-g sample of a certain
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents