In a spectrum chart (like the one shown in figure Q11.2) , the emission lines produced by a quantum harmonic oscillator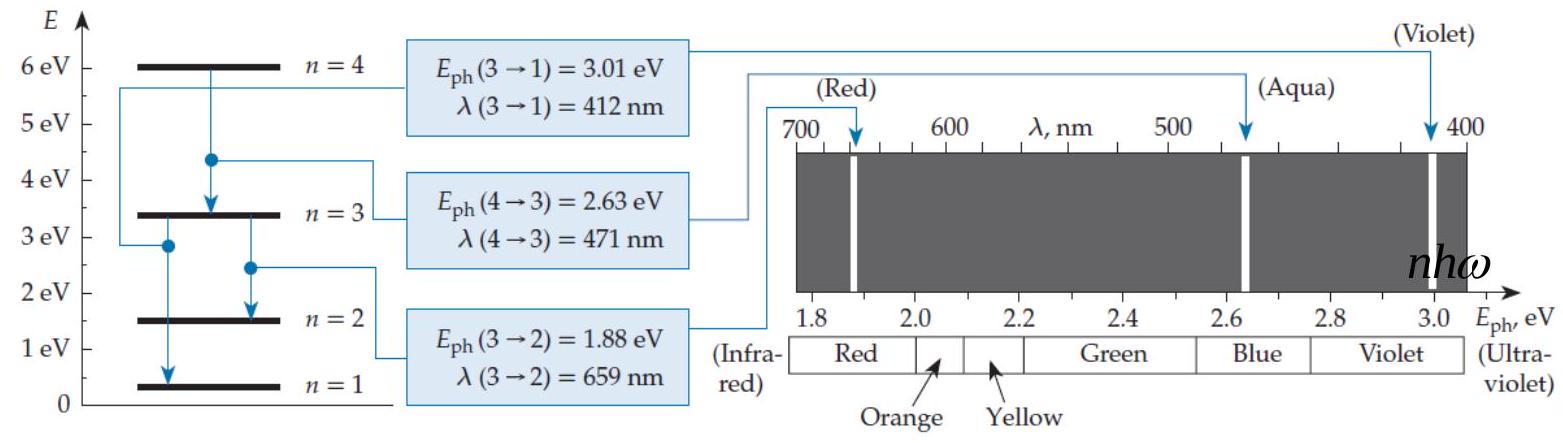
A) Get closer together (in energy) as we go to the right.
B) Are evenly spaced (in energy) .
C) Get farther apart (in energy) as we go to the right.
Correct Answer:
Verified
Q130: Suppose we have a quantum system
Q131: In a cyanine dye molecule, one electron
Q132: A hydrochloric acid molecule consists of a
Q133: A meson is a subatomic "particle" consisting
Q134: Imagine that the energy levels of
Q136: Suppose the energy of the longest-wavelength
Q137: Two electrons are placed into separate
Q138: Suppose we have a vial of quantum
Q139: Suppose we measure the wavelengths of
Q140: Quantum issues often constrain transitions in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents