Why does pyrrole have a lower pKa (weaker base) than pyridine or pyrimidine? 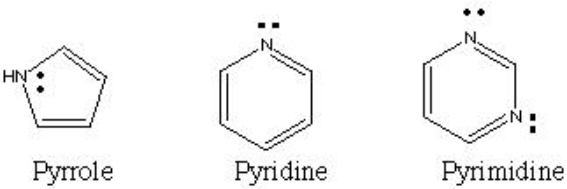
A) Because pyrrole is smaller and more compact and this prevents its lone pair from reacting as much.
B) Because pyrrole has less carbons and therefore the lone pair is more active.
C) Because pyrrole's lone pair participates in the delocalized electron cloud, while pyridine's and pyrimidine's lone pairs do not.
D) Because pyrimidine has more lone pairs than pyrrole does.
Correct Answer:
Verified
Q1: What is the structure of N,N-diethylpentanamine?
Q3: What is the product of the following
Q4: What is the product of the following
Q5: What is the product of the following
Q6: What is the product of the following
Q7: What is the product of the following
Q8: What is the product of the following
Q9: What is the product of the following
Q10: What is the product of the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents