Why, primarily, does tetrahydrofuran (THF, bp 66 °C) have a higher boiling point than cyclopentane (bp 49 °C) ? 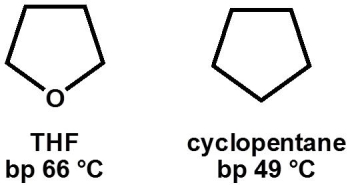
A) increased London dispersion forces
B) hydrogen bonding
C) increased mass
D) decreased London dispersion forces
E) dipole-dipole interactions
Correct Answer:
Verified
Q1: How many of the nine constitutional isomers
Q2: Which of the following names is correct?
Q3: Choose the best name for the following
Q4: Choose the best name for the following
Q5: Choose the best name for the following
Q6: Which of the following is a tertiary
Q7: Which of the following trimethylcyclohexanes has the
Q8: Which of the following dimethylcyclohexanes has the
Q10: Rank the alcohols, all of similar molecular
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents