
Refer to the figure below, and then answer the question that follows.
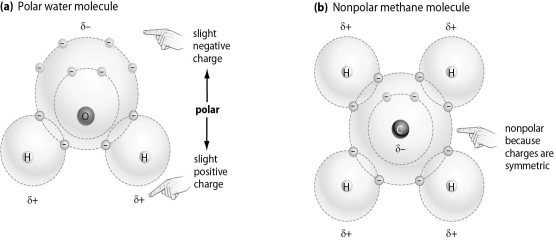
-Which of the following molecules is most likely to bind to an ion,and why?
A) Molecule A, because it has electrical charges that will attract an ion
B) Molecule B, because it has four hydrogen atoms on the exterior of the molecule
C) Molecule A, because any molecule with oxygen is able to bind to an ion
D) Molecule B, because it has a carbon at in the center of the molecule
Correct Answer:
Verified
Q63: A signal molecule will _ to a
Q64: What are the three most important subatomic
Q65: What is chemical bonding? Explain the differences
Q66: Explain how a polar molecule,such as water,can
Q67: Refer to the figure below, and then
Q69: Oil spills in the ocean are often
Q70: You have been having trouble with acid
Q71: It takes more energy to raise the
Q72: Temperatures on the Earth are moderated by
Q73: A(n)_ has a higher pH than a(n)_.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents