
Refer to the figure below, and then answer the question that follows.
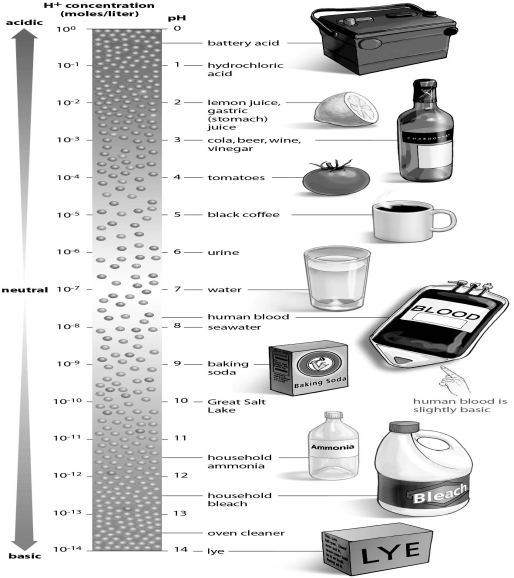
-You are working in a chemistry lab,and your lab partner knocks over a beaker of hydrochloric acid.You alert your laboratory instructor,and he immediately pours another solution over the spill to neutralize the acid.Using the figure as a guide,what did your instructor pour onto the acid to neutralize it?
A) water
B) baking soda
C) lemon juice
D) coffee
Correct Answer:
Verified
Q56: A single covalent chemical bond represents a
Q57: Buffering systems work to maintain pH within
Q58: As an acid mixes in water:
A)the number
Q59: Chemical reactions involve only the outermost electrons
Q60: Which elements make up the majority of
Q62: How are ions formed? Why do ionic
Q63: A signal molecule will _ to a
Q64: What are the three most important subatomic
Q65: What is chemical bonding? Explain the differences
Q66: Explain how a polar molecule,such as water,can
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents