Multiple Choice
Which would you expect to be most soluble in water? 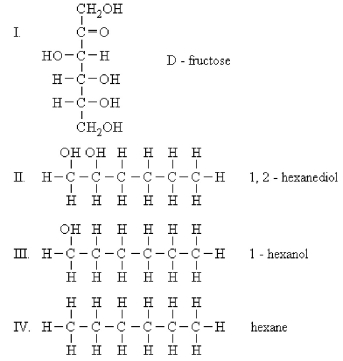
A) I
B) II
C) III
D) IV
Correct Answer:
Verified
Related Questions
Q1: Cells keep the osmotic pressure from being
Q2: Which substance do you expect to be
Q3: The osmotic pressure of a 0.010 M
Q5: Poorly soluble molecules such as lipids and
Q6: A molecule or ion is said to
Q7: The polarity of small molecules is a
Q8: Which statement does NOT explain the polarity
Q9: The osmotic pressure of an aqueous solution
Q10: Solutes diffuse more slowly in cytoplasm than
Q11: Oil and water do not form a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents