If an atom of sulfur (atomic number 16) were allowed to react with atoms of hydrogen (atomic number 1) , which of the molecules below would be formed?
A) S- H
B) H- S -H
C) 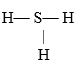
D) 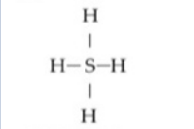
E) H=S=H
Correct Answer:
Verified
Q37: What results from an unequal sharing of
Q42: The ionic bond of sodium chloride is
Q43: What is the difference between covalent bonds
Q44: A van der Waals interaction is the
Q45: A covalent chemical bond is one in
Q48: In ammonium chloride salt (NH₄Cl)the anion is
Q49: Which of the following is not considered
Q51: A molecule of carbon dioxide (CO₂)is formed
Q57: Van der Waals interactions result when
A) hybrid
Q59: Which of the following explains most specifically
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents