The following questions refer to Figure 2.3.
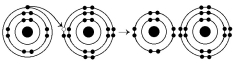
-What results from the chemical reaction illustrated in Figure 2.3?
A) a cation with a net charge of +1
B) a cation with a net charge of -1
C) an anion with a net charge of +1
D) an anion with a net charge of -1
E) A and D
Correct Answer:
Verified
Q26: The atomic number of chlorine is 17.
Q37: What results from an unequal sharing of
Q48: In ammonium chloride salt (NH₄Cl)the anion is
Q49: Which of the following is not considered
Q51: A molecule of carbon dioxide (CO₂)is formed
Q53: A covalent bond is likely to be
Q54: The following questions refer to Figure 2.3.
Q55: Which of the following molecules contains the
Q56: When two atoms are equally electronegative, they
Q57: Van der Waals interactions result when
A) hybrid
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents