The following question is based on Figure 3.1: solute molecule surrounded by a hydration shell of water.
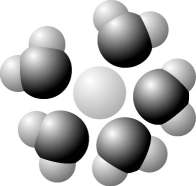
-Based on your knowledge of the polarity of water molecules, the solute molecule is most likely
A) positively charged.
B) negatively charged.
C) without charge.
D) hydrophobic.
E) nonpolar.
Correct Answer:
Verified
Q2: Temperature usually increases when water condenses. Which
Q4: The nutritional information on a cereal box
Q7: The slight negative charge at one end
Q8: Water is able to form hydrogen bonds
Q10: Why does ice float in liquid water?
A)The
Q11: Water's high specific heat is mainly a
Q13: Hydrophobic substances such as vegetable oil are
A)
Q15: An example of a hydrogen bond is
Q16: Which of the following effects is produced
Q17: Which of the following takes place as
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents