Multiple Choice
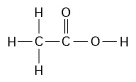
-How many grams of the molecule in Figure 3.2 would be required to make 1 L of a 0.5 M solution of the molecule?
(Carbon = 12, Oxygen = 16, Hydrogen = 1)
A) 29
B) 30
C) 60
D) 150
E) 342
Correct Answer:
Verified
Related Questions
Q20: How many molecules of glycerol (C₃H₈O₃)would be
Q21: The molecular weight of water is 18
Q22: You have a freshly prepared 1 M
Q23: Which of the following statements is completely
Q24: The molecular mass of glucose (C₆H₁₂O₆)is 180
Q26: The molecular mass of glucose is 180
Q28: What is the pH of a solution
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents