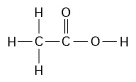
-How many grams of the molecule in Figure 3.2 would be required to make 2.5 L of a 1 M solution of the molecule?
(Carbon = 12, Oxygen = 16, Hydrogen = 1)
A) 29
B) 30
C) 60
D) 150
E) 342
Correct Answer:
Verified
Q34: What is the pH of a solution
Q35: A given solution contains 0.0001(10⁻⁴)moles of hydrogen
Q36: Which of the following solutions has the
Q37: A small birthday candle is weighed, then
Q38: Identical heat lamps are arranged to shine
Q40: If the pH of a solution is
Q41: You have two beakers. One contains a
Q43: Assume that acid rain has lowered the
Q44: Pure, freshly-distilled water has a pH of
Q68: The bonds that are broken when water
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents