Carbon dioxide (CO₂) is readily soluble in water, according to the equation CO₂ + H₂O ↔ H₂CO₃. Carbonic acid (H₂CO₃) is a weak acid. If CO₂ is bubbled into a beaker containing pure, freshly distilled water, which of the following graphs correctly describes the results?
A) 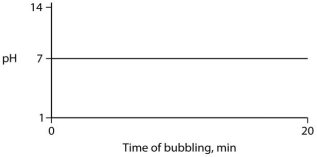
B) 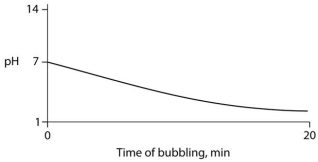
C) 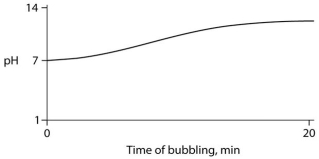
D) 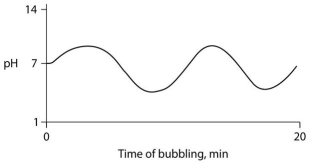
E) 
Correct Answer:
Verified
Q58: Q65: You have two beakers. One contains a Q66: Measurements show that the pH of a Q68: The bonds that are broken when water Q69: Which of the following is a hydrophobic Q70: Use the following information to answer the Q72: Identical heat lamps are arranged to shine Q74: A small birthday candle is weighed, then Q76: Use the following information to answer the Q78: Use the following information to answer the![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents