The following questions are based on the reaction A + B ↔ C + D shown in the figure below.
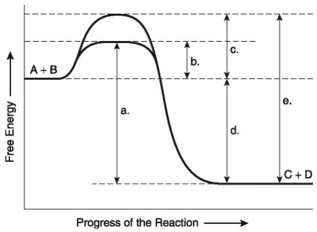
-Which of the following terms best describes the forward reaction in the figure above?
A) endergonic, ∆G > 0
B) exergonic, ∆G < 0
C) endergonic, ∆G < 0
D) exergonic, ∆G > 0
E) chemical equilibrium, ∆G = 0
Correct Answer:
Verified
Q42: Q57: Q63: Succinate dehydrogenase catalyzes the conversion of succinate Q64: The following questions are from the end-of-chapter Q74: Use the following figure to answer the Q76: A chemical agent that speeds up a Q77: The following questions are based on the Q80: The following questions are based on the Q82: Use the following information to answer the Q83: Use the following information to answer the![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents