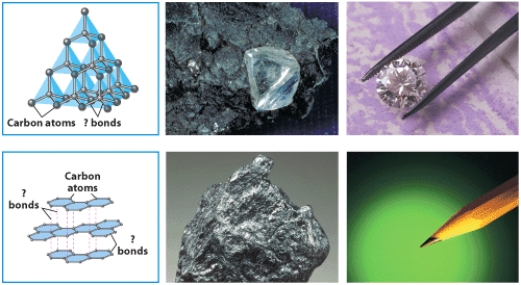
The figure below illustrates bonding in two crystalline structures of carbon.Indicate which one is the structure of graphite and which diamond.Identify the type of bonding in each case and explain how the different bonding in the structures relates to the great difference in hardness between the two minerals.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q62: Discuss some of the ways in which
Q63: Explain why it is not necessary to
Q64: What is the difference between ionic and
Q65: There are 8 protons in the nucleus
Q66: What is the relationship between cleavage,crystal structure,and
Q68: Two chemical elements make up 70 percent
Q69: With approximately 3,500 known minerals,why are there
Q70: Why is color an unreliable property to
Q71: What's the difference between a rock and
Q72: What are the three families of rocks?
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents