in the following model of an atom, draw a transition from the ground state to the first excited state. To draw this, draw the electron's motion as a line with an arrow to indicate direction and draw the photon as a wave with an arrow to indicated direction. Label the electron and photon. Label whether this is an absorption, emission, or continuous spectrum. 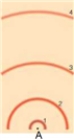
Correct Answer:
Verified
Q115: Blue stars emit most of their energy
Q116: The Lyman series lines of hydrogen all
Q117: Bluer stars are brighter than redder stars
Q118: An atom is ionized if one of
Q119: An atom gained an electron. Therefore it
Q120: Part of astrophysics is understanding the history
Q121: in the following model of an atom,
Q123: What would the spectrum of hydrogen look
Q124: in the following model of an atom,
Q125: Describe two ways in which an atom
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents