An ideal monatomic gas originally in state A is taken reversibly to state B along the straight-line path shown in the pressure-volume graph.
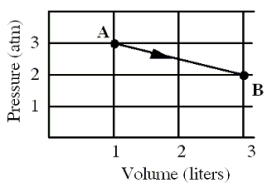
-Suppose that the same gas is originally in state A as described above,but its volume is increased isothermally until a new volume of 3.0 liters is reached.Which one of the following statements for this isothermal process is false?
A) The change in the internal energy is zero.
B) The final state of the system will still be B.
C) The work done will be smaller for the isothermal process.
D) The heat added will be smaller for the isothermal process.
E) The heat added for the isothermal process will be equal to the work done.
Correct Answer:
Verified
Q33: A fixed amount of ideal gas is
Q34: An ideal monatomic gas expands isobarically from
Q35: An ideal monatomic gas originally in state
Q36: An ideal gas is taken from state
Q37: A paddle wheel frictionally adds thermal energy
Q39: A paddle wheel frictionally adds thermal energy
Q40: During one stage of a reversible process,the
Q41: A heat engine operates between a hot
Q42: What change in temperature occurs when 1600
Q43: Which one of the following statements best
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents